Acids, Bases and Salts MCQs:
(b) Baking soda
(c) Washing soda
(d) Gypsum
head>
Hello guys, welcome to your own blog 'Pareeksha Time'. In this article, you will read CBSE Class 10 Chemistry MCQs of Chapter 2. You will read Acids, Bases and Salts MCQs for Term-1 Board Exams 2021-22.
Now Board Exams are divided into two terms; Term-1 & Term-2. In Term-1 Board Exams, only MCQ type questions will be asked.
So you have to practice MCQs of all chapters of your syllabus.
If you want to stay updated to class 10 news and updates, join our Telegram Channel.
To join Telegram Channel, Click on the image below.
Acids, Bases and Salts MCQs|MCQ Questions for Class 10 Chemistry Chapter 2 with Answers:-
1. Which one of the given is incorrect?
(a) Acids turns blue litmus paper red
(b) Aqueous solutions of acids conduct electricity
(c) Acids react with certain metals to form hydrogen gas
(d) None of these
Answer: (d) None of these
2. Which one of the following will turn red litmus blue?
(a) Vinegar
(b) Baking soda solution
(c) Lemon juice
(d) Soft drinks
Answer: (b) Baking soda solution
3. What happens when a solution of an acid is mixed with a solution of a base in a test tube?
(i) Temperature of the solution decreases
(ii) Temperature of the solution increases
(in) Temperature of the solution remains the same
(iv) Salt formation takes place
(a) (i) and (iv)
(b) (i) and (iii)
(c) (ii) only
(d) (ii) and (iv)
Answer: (d) (ii) and (iv)
4. An aqueous solution turns red litmus solution blue. Excess addition of which of the following solution would reverse the change?
(a) Baking powder
(b) Lime
(c) Ammonium hydroxide solution
(d) Hydrochloric acid
Answer: (d) Hydrochloric acid
5. Which of the following is correct about Methyl Orange?
(a) Red in acidic medium, yellow in basic medium
(b) Yellow in acidic medium, pink in basic medium
(c) Colourless in acidic medium, pink in basic medium
(d) Pink in acidic medium, colourless in basic medium
Answer: (a) Red in acidic medium, yellow in basic medium
6. Tomato is a natural source of which acid?
(a) Acetic acid
(b) Citric acid
(c) Tartaric acid
(d) Oxalic acid
Answer: (d) Oxalic acid
7. Which of the following indicators turn red in an acidic solution?
(i) Phenolphthalein
(ii) Litmus
(iii) Turmeric
(iv) Methyl orange
Choose the correct option:
(a) (i) and (ii)
(b) (ii) and (iii)
(c) Only (ii)
(d) (ii) and (iv)
Answer: (d) (ii) and (iv)
8. Which one of the given acids is used in the treatment of bone marrow and scurvy diseases?
(a) Acetic acid
(b) Hydrochloric acid
(c) Ascorbic acid
(d) Nitric acid
Answer: (c) Ascorbic acid
9. Two aqueous solutions P and Q have pH of 5 and 13 respectively. The correct inference is that:
(a) Solution P is of HCl and Q is of NH4OH
(b) Solution P is of CH3COOH and Q is of Ca(OH)2
(c) Solution P is of HNO3 and Q is of NH4OH
(d) Solution P is of CH3COOH and Q is of NaOH
Answer: (d) Solution P is of CH3COOH and Q is of NaOH
10. Which of the following does not form an acidic salt?
(a) Phosphoric acid
(b) Carbonic acid
(c) Hydrochloric acid
(d) Sulphuric acid
Answer: (b) Carbonic acid
11. Alkalis are:
(a) Acids, which are soluble in water
(b) Acids, which are insoluble in water
(c) Bases, which are insoluble in water
(d) Bases, which are soluble in water
Answer: (d) Bases, which are soluble in water
12. The pH of commonly used toothpaste is
(a) <6.5
(b) ≥7.0
(c) ≥2.2
(d) None of these
Answer: (b) ≥7.0
13. The pH of three solutions X, Y and Z is 6, 4 and 8 respectively. Which of the following is the correct order of acidic strength?
(a) X > Y > Z
(b) Z > Y > X
(c) Y > X > Z
(d) Z > X > Y
Answer: (c) Y > X > Z
14. The sample of soil from a particular place was tested for its pH value. It came out to be 5. Which one of the following should be added to the soil to make it suitable for the plant growth?
i. Calcium chloride
ii. Calcium Hydroxide
iii. Calcium oxide
Choose the correct option:
(a) Both (i) and (ii)
(b) Both (ii) and (iii)
(c) Only (i)
(d) Only (iii)
Answer: (b) Both (ii) and (iii)
15. Butyric acid is found in:
(a) Rancid butter
(b) Rancid cake
(c) Stings of bees
(d) All of these
Answer: (b) Rancid cake
16. Which one of the following can be used as an acid-base indicator by a visually impaired student?
(a) Litmus
(b) Turmeric
(c) Vanilla essence
(d) Petunia leaves
Answer: (c) Vanilla essence
17. The function of quick lime in soda lime mixture is to
(a) Absorb moisture present in soda lime
(b) Increase the efficiency of soda lime
(c) Increase the pH of soda lime
(d) Take part in reaction with NaOH
Answer: (a) Absorb moisture present in soda lime
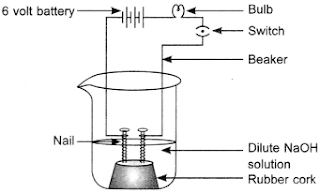
18. The apparatus given in the adjoining figure was set up to demonstrate electrical conductivity.
Which of the following statement(s) is (are) correct?(i) Bulb will not glow because electrolyte is not acidic.
(ii) Bulb will glow because HCl is a strong acid and furnishes ions for conduction.
(iii) Bulb will not glow because circuit is incomplete.
(iv) Bulb will not glow because it depends upon the type of electrolytic solution.
(a) (i) and (iii)
(b) (ii) and (iv)
(c) (ii) only
(d) (iv) only
Answer: (c) (ii) only
19. In the following reaction, identify the salt formed:
NH4OH (aq) + H2SO4 (aq) → _____ + 2H2O (l)
(a) NH4NO3
(b) (NH4)2SO4
(c) (NH4)3PO4
(d) (NH4)2S
Answer: (b) (NH4)2SO4
20. The difference of molecules of water in gypsum and Plaster of Paris (PoP) is:
(a) 5/2
(b) 1
(c) 3/2
(d) ½
Answer: (c) 3/2
21. Tooth enamel is made up of:
(a) Calcium phosphate
(b) Calcium carbonate
(c) Calcium oxide
(d) Potassium
Answer: (a) calcium phosphate
22. Which of the following salts does not contain water of crystallisation?
[NCERT Exemplar Problems]
(a) Blue vitriol(b) Baking soda
(c) Washing soda
(d) Gypsum
Answer: (b) Baking soda
23. Which among the following represents the chemical formula for ‘Plaster of Paris’?
(a) CaSO4.2H2O
(b) CaSO4.5H2O
(c) CaSO4.H2O
(d) CaSO4.1/2H2O
Answer: (d) CaSO4.1/2H2O
24. To protect tooth decay, one is advised to brush the teeth regulary. The ingredient of the paste which checks tooth decay is:
(a) Acidic
(b) Basic
(c) Neutral
(d) Corrosive
Answer: (b) Basic
25. The milkiness (on passing excess carbon dioxide gas through lime water) disappears due to the formation of:
(a) Calcium carbonate
(b) Calcium hydrogen carbonate
(c) Calcium oxide
(d) Calcium Nitrate
Answer: (a) Calcium carbonate
26. Common salt beside being used in the kitchen can also be used as the raw material for the production of:
(i) Baking powder
(ii) Washing soda
(iii) Black ash
(iv) Slaked lime
(a) (ii) and (iii)
(b) (i) and (iii)
(c) (i) and (ii)
(d) (ii) and (iv)
Answer: (c) (i) and (ii)
27. A calcium compound reacts with dilute hydrochloric acid to produce effervescence. The gas evolved extinguishes a burning candle. Identify the compound and the gas evolved:
(a) Calcium Carbonate, Carbon dioxide
(b) Calcium Chloride, Carbon dioxide
(c) Calcium Oxide, Hydrogen
(d) Calcium Carbonate, Hydrogen
Answer: (a) Calcium Carbonate, Carbon dioxide
28. Copper sulphate crystals when heated strongly, lose their water of crystallization to give anhydrous copper sulphate accompanied by a change in color from:
(a) Blue to green
(b) Blue to white
(c) Blue to sky blue
(d) Blue to grey
Answer: (b) Blue to white
So guys here comes end. In this article, I shared 25+ Acids, Bases and Salts MCQs of CBSE Class 10 Chemistry MCQs 2021-22.
Hope you liked this article. Don't forget to join our Telegram Channel to stay updated.
Comment if you liked it.
Thanks.